Using Facebook for Clinical Trial Recruitment

Facebook’s advanced targeting capabilities and enormous user base make it an ideal tool for effective and efficient clinical trial recruitment.
As pharmaceutical companies increasingly turn to digital media for clinical trial recruitment, it’s important they seek out channels that allow for targeted advertising, efficient scaling of ad spend, and advanced campaign performance tracking and optimization capabilities. Facebook’s ad platform checks all three of these boxes, making it an invaluable tool for sponsors and CROs hoping to increase recruitment ROI.
Not only does Facebook allow ad targeting based on condition affinity and demographics, but user interactions with ads (such as “likes” and “shares”) direct the same ads to the user’s friends. Additionally, campaign managers can edit their Facebook ad budget at any time – paired with the company’s Ads Manager app, this provides the ability to continuously optimize your ad spend throughout the course of a trial.
These targeting capabilities complement the obvious advantage of Facebook’s sheer size and popularity. Worldwide, there are over 1.71 billion monthly active Facebook users and 1.13 billion daily active users. (In the U.S. and Canada alone, the number of daily active users is 167 million.) As of June 2016, there were 1.57 billion active mobile users, and this figure is increasing 20% year-over-year. Unsurprisingly, these users translate into pageviews: even back in 2012, approximately 1 in 5 of the total number of pageviews in the U.S. occurred on Facebook.
Ad Types
Facebook provides four placement options for ads – on a user’s Timeline, on their mobile Timeline, on their Instagram account, and in the right column of their desktop or laptop page (see screenshot below). Ads are automatically placed in all four locations, but you can choose to manually place your ads using the Power Editor or Ads Manager. When placing ads manually, try to include a mix of swipe ads and static ads. Since only right column ads are static, marketers should always select this option along with the other placement options.
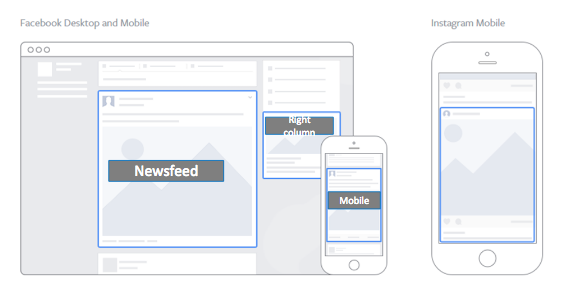
Ads on Facebook are composed of four elements: a header, an image, a call-to-action (CTA), and user engagement options (specifically, Like, Comment, and Share). The ad header, comprised of a page logo, page link, and headline, accounts for about 15% of user responses. The CTA, which is responsible for the other 85%, links to URLs outside of Facebook such as the trial’s patient portal or a local microsite.
Proven Results
For clinical trial recruitment, the industry standard banner ad response rate, or the ratio of click rate to ad impressions, ranges between 0.1% and 0.3% – by comparison, the Facebook ad response rate is between 0.5% and 1.2%, which, considering that the ratio of initial responses to actual enrolled patients is typically high for clinical trials, is a huge advantage. This uptick is likely a result of Facebook’s advanced targeting capabilities, which can direct ads based on location, age, gender, interests, and other qualities that are highly relevant to clinical trial qualification.
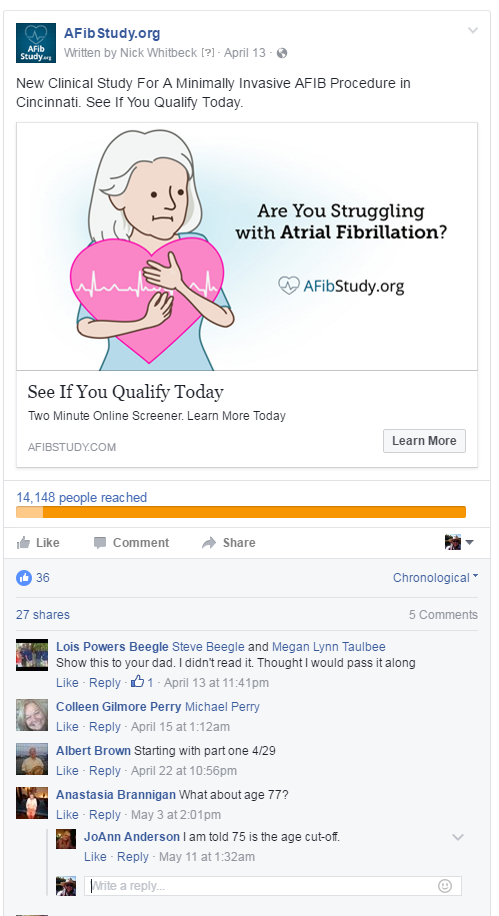
Of course, with so much visibility in a public setting, there’s always the concern of negative user comments and misinformation. Luckily, there are a number of ways to avoid such problems, including disabling user comments, setting up comment moderation, or deleting any potentially harmful or misleading comments before they gain significant visibility. It’s also worth mentioning that of the 3.3 million impressions we served for clinical trial campaigns in the example below, not a single known comment has been posted by an actual trial participant.
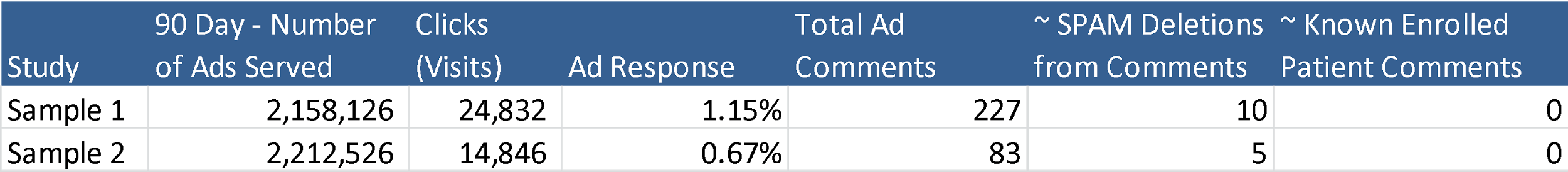
Quite simply, this platform has it all: a massive user base, advanced targeting capabilities, and a number of ways to effectively vet user generated content and maintain control over the trial’s online presence. With so many upsides and so few drawbacks, Facebook advertising for clinical trial recruitment is a no-brainer.

 Back to Blog Home
Back to Blog Home